FDA Clears Nanowear's SimpleSense Non-Invasive Continuous Blood Pressure Monitor

FDA Clears Nanowear's SimpleSense Non-Invasive Continuous Blood Pressure Monitor
Nanowear's remote monitoring device and its SimpleSense platform received FDA 510(k) clearance as a continuous blood pressure monitor.

Nanowear Announces FDA 510(k) Clearance for AI-enabled Continuous

ASCA: A new program for biocompatibility testing

Nanowear scores third FDA clearance for connected vest with remote

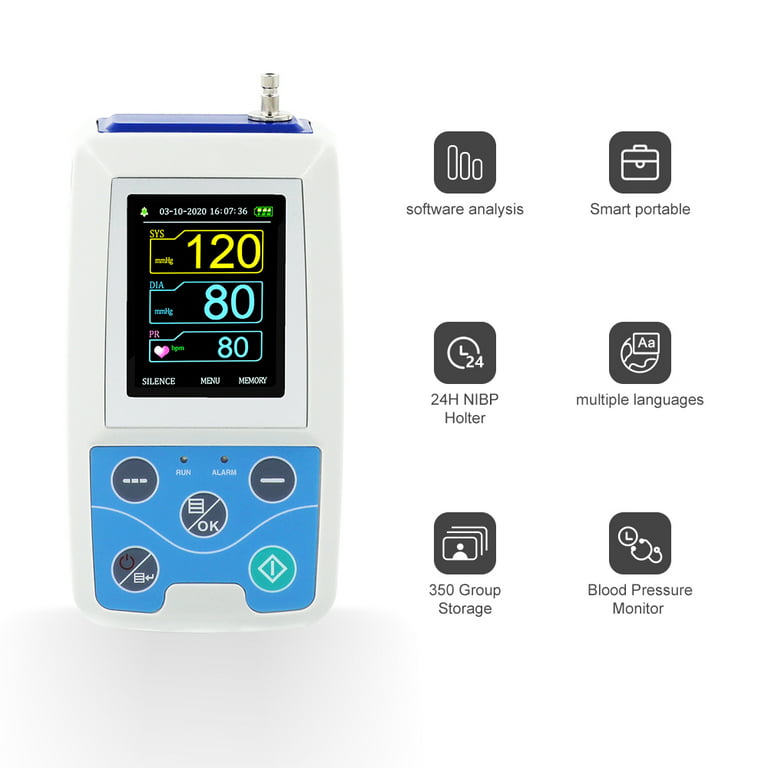
FDA 510(k) clearance for SimpleSense-BP

Finapres® NOVA - Continuous non-invasive hemodynamics!

Nanowear Announces FDA 510(k) Clearance for AI-enabled Continuous
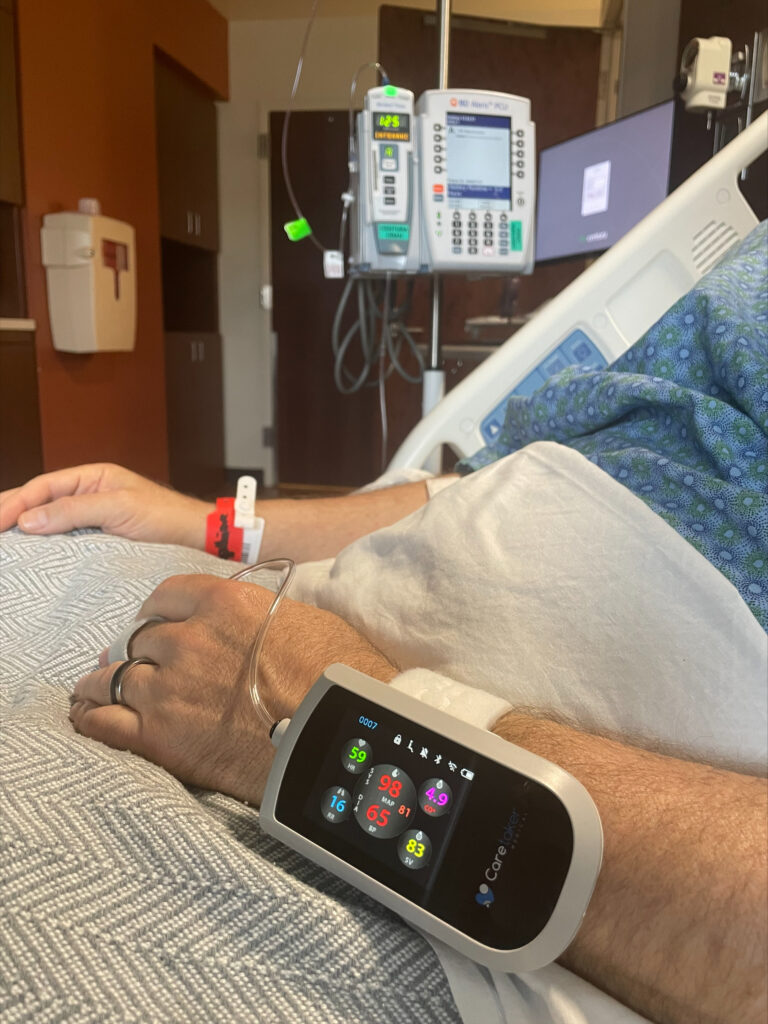
Caretaker Medical's Wireless Monitor is FDA Cleared

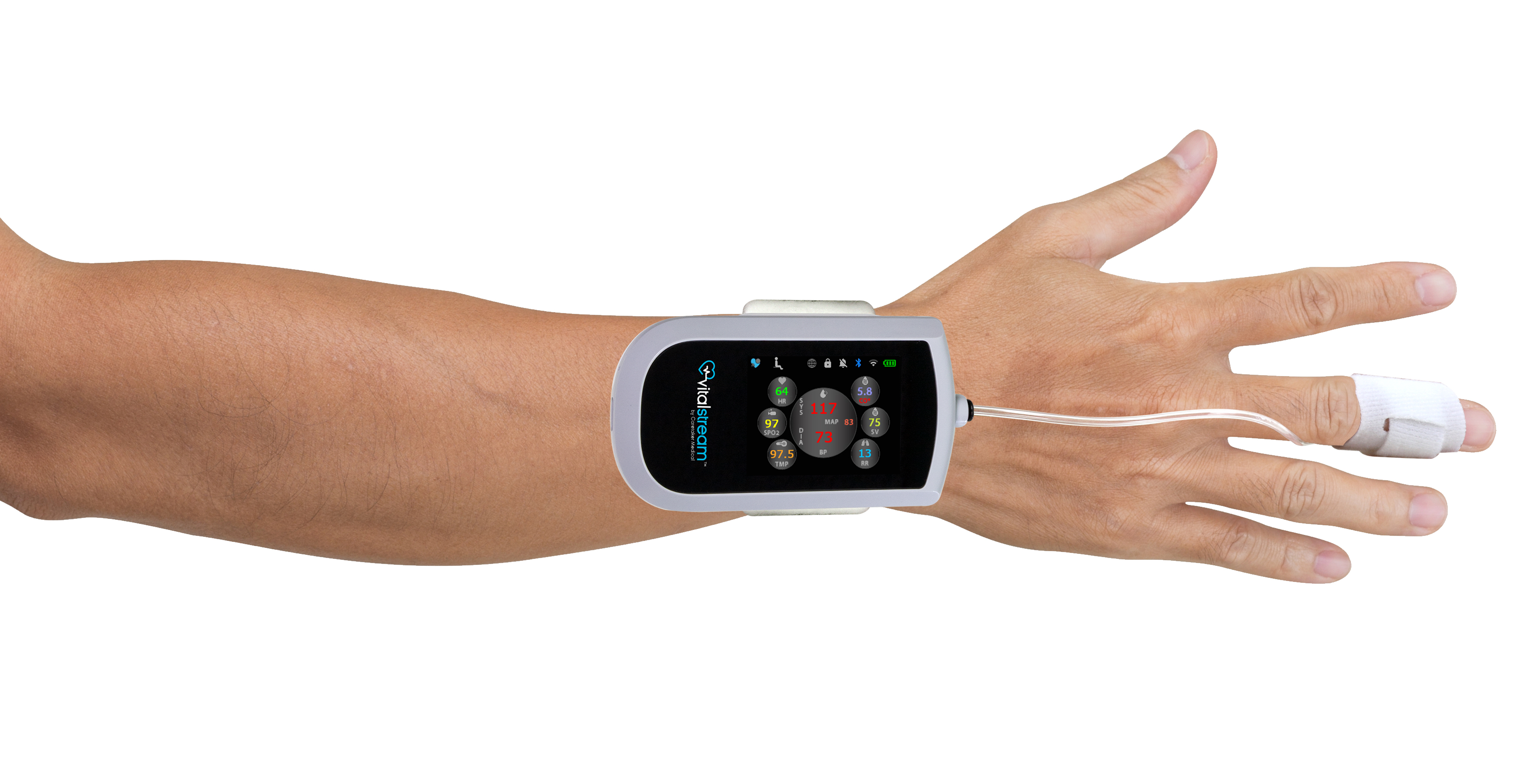
FDA Clears Edwards Lifesciences' Advanced Noninvasive Hemodynamic

Nanowear Announces FDA 510(k) Clearance for AI-enabled Continuous

Commercial Partnership — Nanowear
FDA Clears Caretaker Medical's Wireless Platform for Continuous

Multiparametric cloth-based wearable, SimpleSense, estimates blood
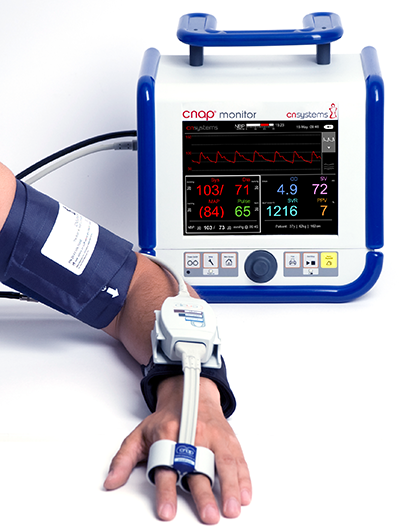
CNAP Monitor 500 HD NEUROSPEC AG Research Neurosciences

Nanowear Announces COVID-19 Remote Diagnostic Research